Qx Therapeutics, Inc receives exclusive license from Yale University on the intellectual property pertinent to the composition, know-how and pre-clinical results generated in Dr Wu’s laboratory on treatment of ALI/ARDS via inhibition of MAP3K2/3.
The technology is applicable to treatment of lung injuries caused by a wide ranges of insults.
The technology can be adapted to treat pulmonary diseases and other acute inflammation-related edema.
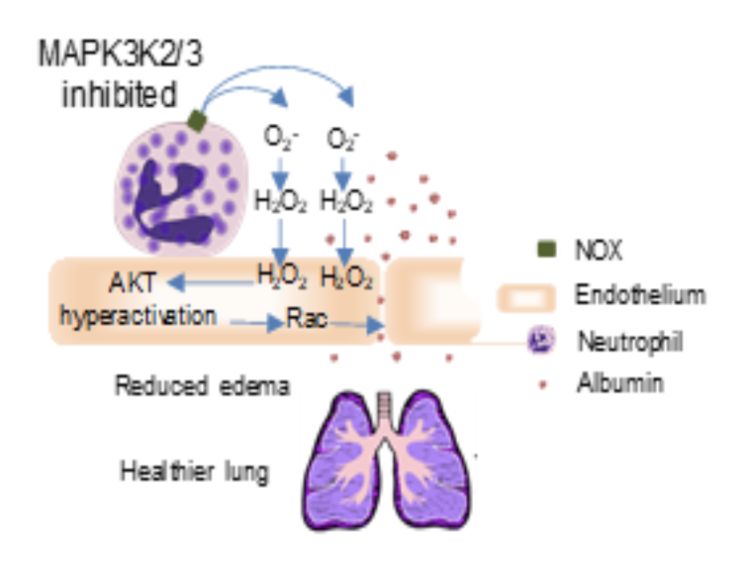
One of the hallmarks of ALI/ARDS is the abundant presence of neutrophils in the lungs. Neutrophils are the most abundant leukocytes in human circulation. They play important roles in innate immunity against microbial infections and contribute to inflammation-related tissue damage. Evidence has clearly shown that neutrophils play important roles in pulmonary edema. While neutrophil extracellular traps and granule enzymes contribute to the pathology of ALI/ARDS, including lung edema.
Studies from Dr. Wu’s lab demonstrated that the Map3k2 and Map3k3 genes were expressed at high levels in mouse neutrophils. In animal ALI model, MAP3K2/3 inhibition significantly reduced permeability and interstitial edema through a moderate increase in neutrophil release of ROS. Importantly, the animal with MAP3K2/3 inhibition had significantly better survival rates than the control animal.
Based on this exciting discovery, we believe our MAP3K2/3 inhibitor, QXT-101, will be a novel effective treatment for ALI patients.